Not long ago, I fought the urge to laugh as my friend smeared a dollop of hand sanitizer all over her orange before peeling and eating it. Was that really necessary? It’s not surprising that bleach wipes, antibiotic mouthwash, and antiseptic wipes are way overused. Scary stories of antibiotic-resistant staph infections ooze from the Ten O’Clock news. Advertisements for household products are so effective that germ-killing agents have become as American as apple pie. Shouldn’t we be worried that using all of these chemicals will kill the benevolent bacteria which live in our guts, breed tremendously hardy pathogens, and prevent us from getting the ambient exposure to pathogens which stimulates our immune systems?
Not long ago, I fought the urge to laugh as my friend smeared a dollop of hand sanitizer all over her orange before peeling and eating it. Was that really necessary? It’s not surprising that bleach wipes, antibiotic mouthwash, and antiseptic wipes are way overused. Scary stories of antibiotic-resistant staph infections ooze from the Ten O’Clock news. Advertisements for household products are so effective that germ-killing agents have become as American as apple pie. Shouldn’t we be worried that using all of these chemicals will kill the benevolent bacteria which live in our guts, breed tremendously hardy pathogens, and prevent us from getting the ambient exposure to pathogens which stimulates our immune systems?
CENTRAL PROCESSING DEPT.

- SGH - CPD staff
- We are an important part of the medical family but seldom seem to be appreciated for what we do. It's our job to make sure the instruments are cleaned and sterilized properly to allow our medical teams to perform surgeries without worry of infection. We also prepackage items that are one-time use. From a doctor's preference card, we pick all the items they have requested to preform each surgery. We also fold and package the linens used to drape patients during surgical procedures. In other areas of the hospital, we supply each floor with sterile and sanitized items they need to complete their functions as well. We are responsible for picking up soiled instruments from each floor twice a day, clean them, reprocess them and then deliver them back to the floors. As you can see our job is an important one. We are proud of the work we do and will continue to work hard to make your medical experiences safe and germ free!!
Saturday, April 10, 2010
FOOD FOR THOUGHT.....
 Not long ago, I fought the urge to laugh as my friend smeared a dollop of hand sanitizer all over her orange before peeling and eating it. Was that really necessary? It’s not surprising that bleach wipes, antibiotic mouthwash, and antiseptic wipes are way overused. Scary stories of antibiotic-resistant staph infections ooze from the Ten O’Clock news. Advertisements for household products are so effective that germ-killing agents have become as American as apple pie. Shouldn’t we be worried that using all of these chemicals will kill the benevolent bacteria which live in our guts, breed tremendously hardy pathogens, and prevent us from getting the ambient exposure to pathogens which stimulates our immune systems?
Not long ago, I fought the urge to laugh as my friend smeared a dollop of hand sanitizer all over her orange before peeling and eating it. Was that really necessary? It’s not surprising that bleach wipes, antibiotic mouthwash, and antiseptic wipes are way overused. Scary stories of antibiotic-resistant staph infections ooze from the Ten O’Clock news. Advertisements for household products are so effective that germ-killing agents have become as American as apple pie. Shouldn’t we be worried that using all of these chemicals will kill the benevolent bacteria which live in our guts, breed tremendously hardy pathogens, and prevent us from getting the ambient exposure to pathogens which stimulates our immune systems?
Sunday, March 28, 2010
DIARY OF A MINOR INTRUMENT SET
1:pm
Big day ahead tomorrow. The weekend was quiet, but this week will be back to the regular grind, with six procedures in four days. I’ve been cleaned and sterilized and am sitting on the case cart, ready to go to the OR. There is a case scheduled for first thing tomorrow morning.
4 p.m.
My case cart is moved to the operating room and left until the procedure begins in the morning. I’m in sterile packaging in a sterile room that is already prepped for the first patient.
Day 2. 9 am.
On my case cart I am wheeled into the operating room where a surgical nurse sets me up. The surgery takes about an hour of my time.
10 a.m.
The case is finished and the patient has been moved to the recovery area. I’m being placed back on the case cart, which is now considered dirty. I’m sent back to the decontamination area, where I just spent part of yesterday. I am sprayed down with an enzematic soak and placed into a soiled instrument transport cart where I wait until someone comes to transport me and the rest of the soiled instruments to the decontamination room downstairs.
10:30 a.m.
I am then sorted and inspected initially, but most instrument sets can be put in a washer, which will go through a sonic-type wash, very similar to a dishwasher. Then I will also be ‘milked’ in there. Milk is a lubrication you put on the instruments. After being lubricated, I am dried, and then I come through on our processing side. It’s about a 30- minute process from when we start hand-washing until it comes out of the washer.
10:45 a.m.
I am now placed carefully in my instrument tray after each part of me is checked to be functioning properly. I am checked for sharpness, pitting or any other damage I may have sustained. Next I am placed in my storage container with a chemical indicator strip and sealed.
11:10 a.m.
I enter the sterilization area. However, there are some other items ahead of me today, so I’m left to wait for a few minutes.
11:30 a.m.
I am placed on a cart and pushed into the sterilizer. Sterilization is a 45-minute process, just using a regular steam sterilizer.
12:20 p.m.
Out of the sterilizer, I’m left to cool for a while.
12:45 p.m.
It’s been 20 minutes, so I’m put onto a transport cart ready to be transported to the sterile supply room.
3:00 p.m.
I am now placed on a shelf until I am needed for the next procedure.
Big day ahead tomorrow. The weekend was quiet, but this week will be back to the regular grind, with six procedures in four days. I’ve been cleaned and sterilized and am sitting on the case cart, ready to go to the OR. There is a case scheduled for first thing tomorrow morning.
4 p.m.
My case cart is moved to the operating room and left until the procedure begins in the morning. I’m in sterile packaging in a sterile room that is already prepped for the first patient.
Day 2. 9 am.
On my case cart I am wheeled into the operating room where a surgical nurse sets me up. The surgery takes about an hour of my time.
10 a.m.
The case is finished and the patient has been moved to the recovery area. I’m being placed back on the case cart, which is now considered dirty. I’m sent back to the decontamination area, where I just spent part of yesterday. I am sprayed down with an enzematic soak and placed into a soiled instrument transport cart where I wait until someone comes to transport me and the rest of the soiled instruments to the decontamination room downstairs.
10:30 a.m.
I am then sorted and inspected initially, but most instrument sets can be put in a washer, which will go through a sonic-type wash, very similar to a dishwasher. Then I will also be ‘milked’ in there. Milk is a lubrication you put on the instruments. After being lubricated, I am dried, and then I come through on our processing side. It’s about a 30- minute process from when we start hand-washing until it comes out of the washer.
10:45 a.m.
I am now placed carefully in my instrument tray after each part of me is checked to be functioning properly. I am checked for sharpness, pitting or any other damage I may have sustained. Next I am placed in my storage container with a chemical indicator strip and sealed.
11:10 a.m.
I enter the sterilization area. However, there are some other items ahead of me today, so I’m left to wait for a few minutes.
11:30 a.m.
I am placed on a cart and pushed into the sterilizer. Sterilization is a 45-minute process, just using a regular steam sterilizer.
12:20 p.m.
Out of the sterilizer, I’m left to cool for a while.
12:45 p.m.
It’s been 20 minutes, so I’m put onto a transport cart ready to be transported to the sterile supply room.
3:00 p.m.
I am now placed on a shelf until I am needed for the next procedure.
BACTERIAL SPORES
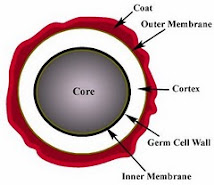
A few species of bacteria have the ability to produce highly resistant structures known as en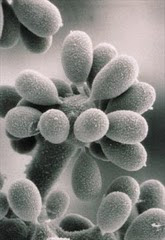 dospores (or simply spores). These resist a range of hazardous environments, and protect against heat, radiation, and desiccation. Endospores form within (hence endo-) special vegetative cells known as sporangia (singular sporangium). Diseases caused by sporing bacteria include botulism (Clostridium botulinum), gas gangrene (Clostridium perfringens), tetanus (Clostridium tetani) and acute food poisoning (Clostridium perfringens, again) All these bacteria are 'anaerobic'. The aerobic sporing bacteria can also cause disease. Anthrax is caused by Bacillus anthracis. Bacillus cereus causes two types of food poisoning.
dospores (or simply spores). These resist a range of hazardous environments, and protect against heat, radiation, and desiccation. Endospores form within (hence endo-) special vegetative cells known as sporangia (singular sporangium). Diseases caused by sporing bacteria include botulism (Clostridium botulinum), gas gangrene (Clostridium perfringens), tetanus (Clostridium tetani) and acute food poisoning (Clostridium perfringens, again) All these bacteria are 'anaerobic'. The aerobic sporing bacteria can also cause disease. Anthrax is caused by Bacillus anthracis. Bacillus cereus causes two types of food poisoning.
 dospores (or simply spores). These resist a range of hazardous environments, and protect against heat, radiation, and desiccation. Endospores form within (hence endo-) special vegetative cells known as sporangia (singular sporangium). Diseases caused by sporing bacteria include botulism (Clostridium botulinum), gas gangrene (Clostridium perfringens), tetanus (Clostridium tetani) and acute food poisoning (Clostridium perfringens, again) All these bacteria are 'anaerobic'. The aerobic sporing bacteria can also cause disease. Anthrax is caused by Bacillus anthracis. Bacillus cereus causes two types of food poisoning.
dospores (or simply spores). These resist a range of hazardous environments, and protect against heat, radiation, and desiccation. Endospores form within (hence endo-) special vegetative cells known as sporangia (singular sporangium). Diseases caused by sporing bacteria include botulism (Clostridium botulinum), gas gangrene (Clostridium perfringens), tetanus (Clostridium tetani) and acute food poisoning (Clostridium perfringens, again) All these bacteria are 'anaerobic'. The aerobic sporing bacteria can also cause disease. Anthrax is caused by Bacillus anthracis. Bacillus cereus causes two types of food poisoning.DUTIES OF A CPD TECH
Sterile processing technicians' primary responsibility is infection control. Their duties vary from one position to another but, in general, they:
* sort, disassemble, clean and disinfect trays, instruments, carts, supplies and equipment
* select and use appropriate cleaning methods
* load, operate and maintain cleaning, disinfecting and sterilizing equipment
* perform standard tests to monitor the effectiveness of sterilization procedures
* sort, assemble and package medical/surgical instruments and equipment
* report damaged or malfunctioning equipment and supplies
* store and rotate sterilized items
* provide instrument sets for surgical procedures and case carts for booked and
emergency surgery
* inventory, select and replenish supplies to medical/surgical carts on a regular basis
monitor quota levels and changes in demand levels
* make appropriate substitutions when necessary and report problems regarding
availability of instruments and supplies
* use computers to order supplies, and process and maintain records
* communicate with operating room personnel to provide required instruments and
surgical supplies.
* sort, disassemble, clean and disinfect trays, instruments, carts, supplies and equipment
* select and use appropriate cleaning methods
* load, operate and maintain cleaning, disinfecting and sterilizing equipment
* perform standard tests to monitor the effectiveness of sterilization procedures
* sort, assemble and package medical/surgical instruments and equipment
* report damaged or malfunctioning equipment and supplies
* store and rotate sterilized items
* provide instrument sets for surgical procedures and case carts for booked and
emergency surgery
* inventory, select and replenish supplies to medical/surgical carts on a regular basis
monitor quota levels and changes in demand levels
* make appropriate substitutions when necessary and report problems regarding
availability of instruments and supplies
* use computers to order supplies, and process and maintain records
* communicate with operating room personnel to provide required instruments and
surgical supplies.
Subscribe to:
Comments (Atom)
STERILE PROCESSING DEPARTMENT
DECONTAMINATION PROCESS
Decontamination is the physical or chemical process that renders an inanimate object that may be contaminated with harmful microbial life safe for further handling. The objective of decontamination is to protect the preparation and package workers who come in contact with medical devices after the decontamination process from contracting diseases caused by microorganisms on those devices.
Steps in the Decontamination Process
Transport - Used supplies and equipment should be collected and taken to the Decontamination Area in the Sterile Processing Department in a way that avoids contamination of personnel or any area of the hospital. Equipment should be covered and supplies should be moved in covered carts, closed totes or containers, or closed plastic bags.
Attire - Personnel working in the decontamination area should wear protective clothing, which includes a scrub uniform covered by a moisture-resistant barrier, shoe covers, rubber or plastic gloves, and a hair covering. During manual cleaning processes, when splashing can occur, safety goggles and a face mask should be worn.
Sorting - sorting begins at the point of use. Handling of contaminated items should be minimized unless the user of the device is already wearing full personal protective attire, such as following care in the operating room. In areas where workers are wearing no or minimal protective attire, sorting should consist only of removing disposable sharps and discarding other single-use items.
Soaking - this is necessary only if you have lumens or other complex designs that are filled with debris or if the devices are very bloody and cannot be rinsed or wiped at the point of use.
Detergent - should be compatible with the materials in the device and suited for the type of soil. Consult the recommendations from the device manufacturer.
equipment - many types of cleaning equipment are available, the most commonly used are:
Washer/decontaminator - the washer/decontaminator is used to clean heat-tolerant items. The cycle consists of several washes and rinses, followed by a steam sterilization cycle appropriate for the types of items contained in the load. Although subjected to a cycle designed to sterilize clean items, items processed in a washer/decontaminator should not be assumed to be sterile at the end of the process. The reason for this is that items enter the washer/decontaminator with an unknown, but probably very high, level of microbial contamination, which the sterilization cycle may not be able to completely destroy.
Ultrasonic - the ultrasonic washer is used to remove fine soil from surgical instruments after manual cleaning and before sterilization. The equipment works by converting high-frequency sound waves into mechanical vibrations that free soil from the surface of instruments. The high-frequency energy causes microscopic bubbles to form on the surface of the instruments and as the bubbles implode, minute vacuum areas are created, drawing out the tiniest particles of debris from the crevices of the instruments. This process is called cavitation.
Tunnel washers - they resemble a mini car-wash. The chief advantage of these units is that most of them allow totally hands-off processing. Instruments in perforated or mesh-bottom trays can come directly from the operating room or other department and be placed into the tunnel washer without any further handling or arranging. Inside, the instruments are subjected to cycles of pre-rinse, washing, ultrasonic, rinse, and drying.
Cart washers - carts and other transportation vehicles and containers must be cleaned routinely to remove dust and spillage. Cartwashers have wash, rinse, steam and drying cycles. Carts are placed in the washer in a tilted position to enable water to drain out and prevent restriction of any moving parts within the washer. Items removed from this type of washer are very hot and must be allowed to cool before they are handled. Carts must be thoroughly dried before they have contact with clean or sterile supplies.
Inspection - after cleaning, all instruments should undergo inspection before being packaged for reuse or storage. Box locks, serrations, and crevices should be critically inspected for cleanliness.
Instruments with cutting edges such as scissors, rongeurs, chisels, curettes, etc., should be checked for sharpness. There should be no dull spots, chips, or dents.
Hinged instruments such as clamps and forceps should be checked for stiffness and alignment of jaws and teeth. Tips should be properly aligned, jaws should meet perfectly, and joints should move easily. Ratchets should close easily and hold firmly. Any instruments with pins or screws should be inspected to make sure they are intact. Plated instruments should be checked to make sure there are no chips, worn spots, or sharp edges. Worn spots can rust during autoclaving. Chipped plating can harbor soil and damage tissue and rubber gloves. If any problems are noticed during the inspection process, these instruments should be either cleaned again, or sent for repair depending on the problem observed.
Steps in the Decontamination Process
Transport - Used supplies and equipment should be collected and taken to the Decontamination Area in the Sterile Processing Department in a way that avoids contamination of personnel or any area of the hospital. Equipment should be covered and supplies should be moved in covered carts, closed totes or containers, or closed plastic bags.
Attire - Personnel working in the decontamination area should wear protective clothing, which includes a scrub uniform covered by a moisture-resistant barrier, shoe covers, rubber or plastic gloves, and a hair covering. During manual cleaning processes, when splashing can occur, safety goggles and a face mask should be worn.
Sorting - sorting begins at the point of use. Handling of contaminated items should be minimized unless the user of the device is already wearing full personal protective attire, such as following care in the operating room. In areas where workers are wearing no or minimal protective attire, sorting should consist only of removing disposable sharps and discarding other single-use items.
Soaking - this is necessary only if you have lumens or other complex designs that are filled with debris or if the devices are very bloody and cannot be rinsed or wiped at the point of use.
Detergent - should be compatible with the materials in the device and suited for the type of soil. Consult the recommendations from the device manufacturer.
equipment - many types of cleaning equipment are available, the most commonly used are:
Washer/decontaminator - the washer/decontaminator is used to clean heat-tolerant items. The cycle consists of several washes and rinses, followed by a steam sterilization cycle appropriate for the types of items contained in the load. Although subjected to a cycle designed to sterilize clean items, items processed in a washer/decontaminator should not be assumed to be sterile at the end of the process. The reason for this is that items enter the washer/decontaminator with an unknown, but probably very high, level of microbial contamination, which the sterilization cycle may not be able to completely destroy.
Ultrasonic - the ultrasonic washer is used to remove fine soil from surgical instruments after manual cleaning and before sterilization. The equipment works by converting high-frequency sound waves into mechanical vibrations that free soil from the surface of instruments. The high-frequency energy causes microscopic bubbles to form on the surface of the instruments and as the bubbles implode, minute vacuum areas are created, drawing out the tiniest particles of debris from the crevices of the instruments. This process is called cavitation.
Tunnel washers - they resemble a mini car-wash. The chief advantage of these units is that most of them allow totally hands-off processing. Instruments in perforated or mesh-bottom trays can come directly from the operating room or other department and be placed into the tunnel washer without any further handling or arranging. Inside, the instruments are subjected to cycles of pre-rinse, washing, ultrasonic, rinse, and drying.
Cart washers - carts and other transportation vehicles and containers must be cleaned routinely to remove dust and spillage. Cartwashers have wash, rinse, steam and drying cycles. Carts are placed in the washer in a tilted position to enable water to drain out and prevent restriction of any moving parts within the washer. Items removed from this type of washer are very hot and must be allowed to cool before they are handled. Carts must be thoroughly dried before they have contact with clean or sterile supplies.
Inspection - after cleaning, all instruments should undergo inspection before being packaged for reuse or storage. Box locks, serrations, and crevices should be critically inspected for cleanliness.
Instruments with cutting edges such as scissors, rongeurs, chisels, curettes, etc., should be checked for sharpness. There should be no dull spots, chips, or dents.
Hinged instruments such as clamps and forceps should be checked for stiffness and alignment of jaws and teeth. Tips should be properly aligned, jaws should meet perfectly, and joints should move easily. Ratchets should close easily and hold firmly. Any instruments with pins or screws should be inspected to make sure they are intact. Plated instruments should be checked to make sure there are no chips, worn spots, or sharp edges. Worn spots can rust during autoclaving. Chipped plating can harbor soil and damage tissue and rubber gloves. If any problems are noticed during the inspection process, these instruments should be either cleaned again, or sent for repair depending on the problem observed.
DECONTAM ROOM

AUTOCLAVE OR STEAM STERILIZER

STERILIZATION PROCESS
Bacterial spores are the most resistant of all living organisms because of their capacity to withstand external destructive agents. Although the physical or chemical process by which all pathogenic and nonpathogenic microorganisms, including spores, are destroyed is not absolute, supplies and equipment are considered sterile when necessary conditions have been met during a sterilization process.
Methods
Reliable sterilization depends on contact of the sterilizing agent with all surfaces of the item to be sterilized. Selection of the agent to achieve sterility depends primarily upon the nature of the item to be sterilized. Time required to kill spores in the equipment available for the process then becomes critical.
Steam
Heat destroys microorganisms, but this process is hastened by the addition of moisture. Steam in itself is inadequate for sterilization. Pressure, greater than atmospheric, is necessary to increase the temperature of steam for thermal destruction of microbial life. Death by moist heat in the form of steam under pressure is caused by the denaturation and coagulation of protein or the enzyme-protein system within the cells. These reactions are catalyzed by the presence of water. Steam is water vapor; it is saturated when it contains a maximum amount of water vapor.
Direct saturated steam contact is the basis of the steam process. Steam, for a specified time at required temperature, must penetrate every fiber and reach every surface of items to be sterilized. When steam enters the sterilizer chamber under pressure, it condenses upon contact with cold items. This condensation liberates heat, simultaneously heating and wetting all items in the load, thereby providing the two requisites: moisture and heat.
No living thing can survive direct exposure to saturated steam at 250 F (120 C) longer than 15 minutes. As temperature is increased, time may be decreased. A minimum temperature-time relationship must be maintained throughout all portions of load to accomplish effective sterilization. Exposure time depends upon size and contents of load, and temperature within the sterilizer. At the end of the cycle, re-evaporation of water condensate must effectively dry contents of the load to maintain sterility.
Hydrogen peroxide plasma - hydrogen peroxide is activated to create a reactive plasma or vapor. Plasma is a state of matter distinguishable from solid, liquid, or gas. It can be produced through the action of either a strong electric or magnetic field, somewhat like a neon light. The cloud of plasma created consists of ions, electrons, and neutral atomic particles that produce a visible glow. Free radicals of the hydrogen peroxide in the cloud interact with the cell membranes, enzymes, or nucleic acids to disrupt life functions of microorganisms. The plasma and vapor phases of hydrogen peroxide are highly sporicidal even at low concentrations and temperature.
Methods
Reliable sterilization depends on contact of the sterilizing agent with all surfaces of the item to be sterilized. Selection of the agent to achieve sterility depends primarily upon the nature of the item to be sterilized. Time required to kill spores in the equipment available for the process then becomes critical.
Steam
Heat destroys microorganisms, but this process is hastened by the addition of moisture. Steam in itself is inadequate for sterilization. Pressure, greater than atmospheric, is necessary to increase the temperature of steam for thermal destruction of microbial life. Death by moist heat in the form of steam under pressure is caused by the denaturation and coagulation of protein or the enzyme-protein system within the cells. These reactions are catalyzed by the presence of water. Steam is water vapor; it is saturated when it contains a maximum amount of water vapor.
Direct saturated steam contact is the basis of the steam process. Steam, for a specified time at required temperature, must penetrate every fiber and reach every surface of items to be sterilized. When steam enters the sterilizer chamber under pressure, it condenses upon contact with cold items. This condensation liberates heat, simultaneously heating and wetting all items in the load, thereby providing the two requisites: moisture and heat.
No living thing can survive direct exposure to saturated steam at 250 F (120 C) longer than 15 minutes. As temperature is increased, time may be decreased. A minimum temperature-time relationship must be maintained throughout all portions of load to accomplish effective sterilization. Exposure time depends upon size and contents of load, and temperature within the sterilizer. At the end of the cycle, re-evaporation of water condensate must effectively dry contents of the load to maintain sterility.
Hydrogen peroxide plasma - hydrogen peroxide is activated to create a reactive plasma or vapor. Plasma is a state of matter distinguishable from solid, liquid, or gas. It can be produced through the action of either a strong electric or magnetic field, somewhat like a neon light. The cloud of plasma created consists of ions, electrons, and neutral atomic particles that produce a visible glow. Free radicals of the hydrogen peroxide in the cloud interact with the cell membranes, enzymes, or nucleic acids to disrupt life functions of microorganisms. The plasma and vapor phases of hydrogen peroxide are highly sporicidal even at low concentrations and temperature.

ASSEMBLY AND PACKAGING PROCESS
After the instruments have been cleaned and inspected, they are typically assembled into sets or trays according to detailED instructions for assembling each set or tray.
Instruments and other items that are prepared for sterilization must be packaged so that their sterility can be maintained to the point of use. The materials and techniques used for packaging must allow the sterilant to contact the device during the sterilization process as well as to protect the device from contamination during storage and handling before it is used. The time between sterilization and use may range from a few minutes to several weeks to many months. The packaging material selected must also permit the device to be removed aseptically.
Types of Packaging:
* textiles
* nonwovens
* pouch packaging
* rigid container systems
THE CYCLE CONTINUES

ADMINISTRATIVE MONITORING
Work practices must be supervised. Written policies and procedures must be strictly followed by all personnel responsible and accountable for sterilizing and disinfecting items, and for handling sterile supplies. If sterility cannot be achieved or maintained, the system has failed. Policies and procedures pertain to;
Decontaminating, terminally sterilizing, and cleaning all reusable items; disposing of disposable items.
Packaging and labeling of items.
Loading and unloading the sterilizer.
Operating the sterilizer.
Monitoring and maintaining records of each cycle.
Adhering to safety precautions and preventive maintenance protocol.
Storing of sterile items.
Handling sterile items ready for use.
Making sterile transfer to a sterile field.
Mechanical Indicators
Sterilizers have gauges, thermometers, timers, recorders, and/or other devices that monitor their functions. Most sterilizers have automatic controls and locking devices. Some have alarm systems that are activated if the sterilizer fails to operate correctly. Records are maintained and review for each cycle. Test packs (Bowie-Dick test) are run at least daily to monitor functions of each sterilizer, as appropriate. These can identify process errors in packing or loading.
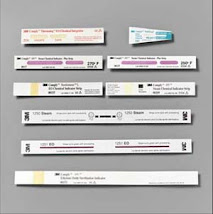
Chemical Indicators
A chemical indicator on a package verifies exposure to a sterilization process. An indicator should be clearly visible on the outside of every on-site sterilized package. This helps differentiate sterilized from unsterilized items. More importantly, it helps monitor physical conditions within the sterilizer to alert personnel if the process has been inadequate. An indicator may be placed inside a package in a position most likely to be difficult for the sterilant to penetrate. A chemical indicator can detect sterilizer malfunction or human error in packaging or loading the sterilizer. If a chemical reaction on the indicator does not show expected results, the item should not be used. Several types of chemical indicators are available:
Tape, labels, and paper strips printed with an ink that changes color when exposed to one or more process parameters.
Glass tube with pellets that melts when a specific temperature is attained in sterilizer.
Integrating or wicking paper with an ink or chemical tablet at one end that melts and wicks along paper over time under desired process parameters. The color bar reaches the "accept" area if parameters are met.
Biological Indicators
Positive assurance that sterilization conditions have been achieved can be obtained only through a biologic control test. The biologic indicator detects nonsterilizing conditions in the sterilizer. A biologic indicator is a preparation of living spores resistant to the sterilizing agent. These may be supplied in a self-contained system, in dry spore strips or discs in envelopes, or sealed vials or ampoules of spores to be sterilized and a control that is not sterilized. Some incorporate a chemical indicator also. The sterilized units and the control are incubated for 24 hours for Bacillus stearothermophilis at 131 to 141 F (55 to 66 C) to test steam under pressure, for 48 hours for Bacillus Subtilis at 95 to 98.6 F (35 to 37 C) to test ethylene oxide.
A biologic indicator must conform with USP testing standards. A control test must be performed at least weekly in each sterilizer. Many hospitals monitor on a daily basis; others test each cycle. Very load of implantable devices must be monitored and the implant should not be used until negative test results are known. Biological indicators also are used as a challenge test before introducing new products or packaging materials, after major repairs on the sterilizer, or after a sterilization failure. All test results are filled as a permanent record for each sterilizer.
Decontaminating, terminally sterilizing, and cleaning all reusable items; disposing of disposable items.
Packaging and labeling of items.
Loading and unloading the sterilizer.
Operating the sterilizer.
Monitoring and maintaining records of each cycle.
Adhering to safety precautions and preventive maintenance protocol.
Storing of sterile items.
Handling sterile items ready for use.
Making sterile transfer to a sterile field.
Mechanical Indicators
Sterilizers have gauges, thermometers, timers, recorders, and/or other devices that monitor their functions. Most sterilizers have automatic controls and locking devices. Some have alarm systems that are activated if the sterilizer fails to operate correctly. Records are maintained and review for each cycle. Test packs (Bowie-Dick test) are run at least daily to monitor functions of each sterilizer, as appropriate. These can identify process errors in packing or loading.
Chemical Indicators
A chemical indicator on a package verifies exposure to a sterilization process. An indicator should be clearly visible on the outside of every on-site sterilized package. This helps differentiate sterilized from unsterilized items. More importantly, it helps monitor physical conditions within the sterilizer to alert personnel if the process has been inadequate. An indicator may be placed inside a package in a position most likely to be difficult for the sterilant to penetrate. A chemical indicator can detect sterilizer malfunction or human error in packaging or loading the sterilizer. If a chemical reaction on the indicator does not show expected results, the item should not be used. Several types of chemical indicators are available:
Tape, labels, and paper strips printed with an ink that changes color when exposed to one or more process parameters.
Glass tube with pellets that melts when a specific temperature is attained in sterilizer.
Integrating or wicking paper with an ink or chemical tablet at one end that melts and wicks along paper over time under desired process parameters. The color bar reaches the "accept" area if parameters are met.
Biological Indicators
Positive assurance that sterilization conditions have been achieved can be obtained only through a biologic control test. The biologic indicator detects nonsterilizing conditions in the sterilizer. A biologic indicator is a preparation of living spores resistant to the sterilizing agent. These may be supplied in a self-contained system, in dry spore strips or discs in envelopes, or sealed vials or ampoules of spores to be sterilized and a control that is not sterilized. Some incorporate a chemical indicator also. The sterilized units and the control are incubated for 24 hours for Bacillus stearothermophilis at 131 to 141 F (55 to 66 C) to test steam under pressure, for 48 hours for Bacillus Subtilis at 95 to 98.6 F (35 to 37 C) to test ethylene oxide.
A biologic indicator must conform with USP testing standards. A control test must be performed at least weekly in each sterilizer. Many hospitals monitor on a daily basis; others test each cycle. Very load of implantable devices must be monitored and the implant should not be used until negative test results are known. Biological indicators also are used as a challenge test before introducing new products or packaging materials, after major repairs on the sterilizer, or after a sterilization failure. All test results are filled as a permanent record for each sterilizer.